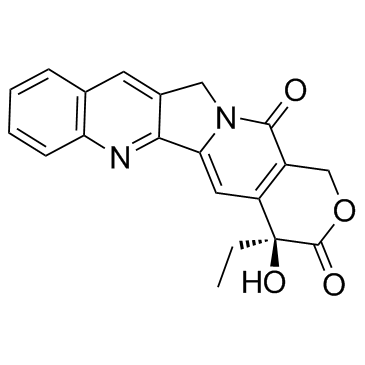
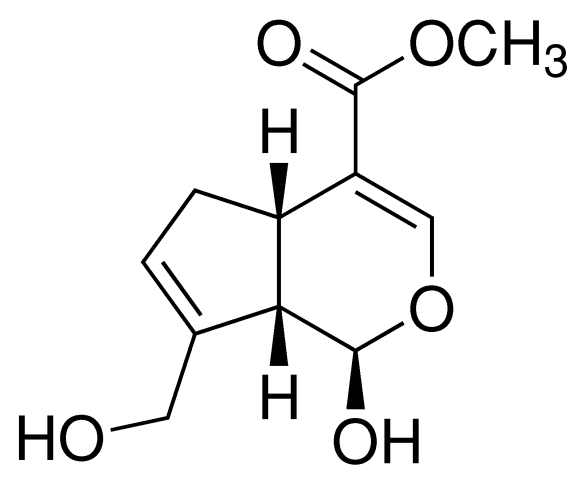
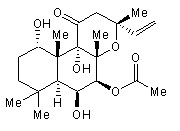
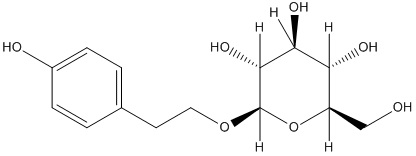
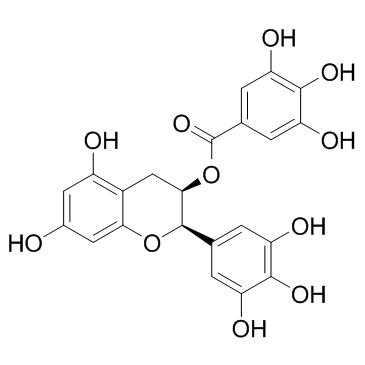
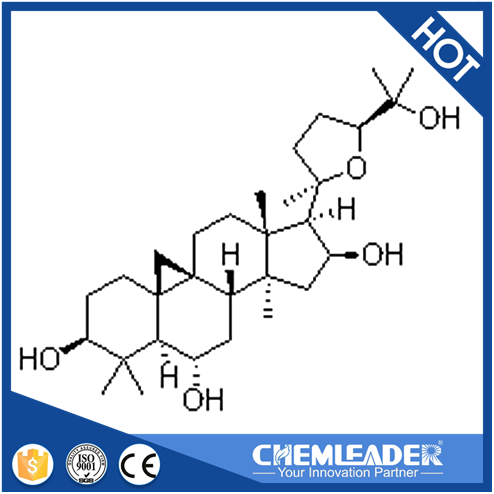
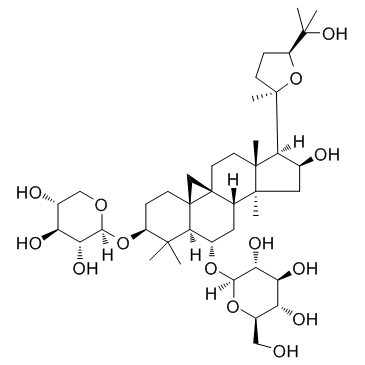
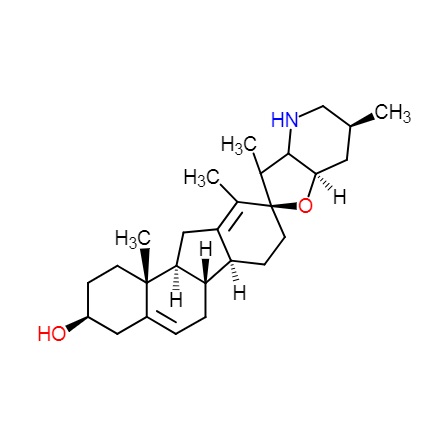
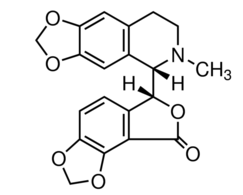
DNA topoisomerases relax DNA torsional strain created during replication, transcription, recombination, repair, and chromosome condensation. The relaxation of DNA supercoiling by topoisomerase I at single-strand breaks enables anticancer agents to reversibly trap the complex by intercalating between DNA base pairs at the cleavage site, thus inhibiting religation, which activates apoptotic and cell cycle arrest pathways.Camptothecin is a cytotoxic, quinoline alkaloid, discovered as the active principle of extracts from the Chinese tree C. acuminate, that inhibits the DNA enzyme topoisomerase I (Top1). It binds the Top1-DNA cleavage complex, inducing DNA-strand breaks. Camptothecin has strong anti-tumor activity against a wide range of experimental tumors and inhibits both DNA and RNA synthesis in mammalian cells. It displays cytotoxity in HT-29 cells with an IC50 value of 10 nM and induces DNA damage at concentrations as low as 51 nM in whole cells and 12 nM in isolated nuclei in in vitro assays.
Statement:
->>Products covered by valid patents are not offered or supplied for commercial use.
->>Products currently covered by valid US Patents are offered for laboratory R&D use in accordance with 35 USC 271(e)+A13(1).
->>Any patent infringement and resulting liability is solely at buyer's risk.
->>Our products are only raw materials for drugs or industry, can't be used for human or animals directly.