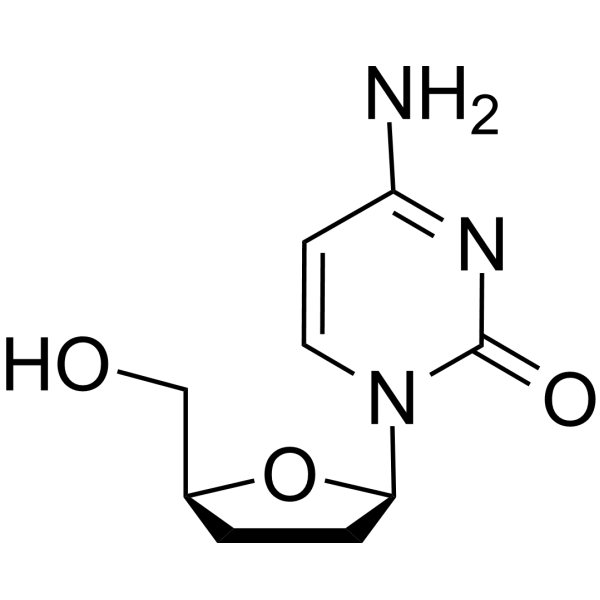
Zalcitabine (trade name Hivid) is a nucleoside analog HIV reverse transcriptase inhibitor (NARTI). It is an antiviral pyrimidine nucleoside analogue effective against HIV replication. 2′,3′-Dideoxycytidine, when activated to its triphosphate, is incorporated into DNA by HIV-1 RT (HIV-1 reverse transcriptase), causing DNA chain termination and viral replication. Zalcitabine was the third antiretroviral to be approved by the Food and Drug Administration (FDA) for the treatment of HIV/AIDS. It is used as part of a combination regimen. Zalcitabine appears less potent than some other nucleoside RTIs, has an inconvenient three-times daily frequency and is associated with serious adverse events. For these reasons it is now rarely used to treat human immunodeficiency virus (HIV), and it has even been removed from pharmacies entirely in some countries.
Statement:
->>Products covered by valid patents are not offered or supplied for commercial use.
->>Products currently covered by valid US Patents are offered for laboratory R&D use in accordance with 35 USC 271(e)+A13(1).
->>Any patent infringement and resulting liability is solely at buyer's risk.
->>Our products are only raw materials for drugs or industry, can't be used for human or animals directly.